Objective
The objective of this experiment was to use respirometers to measure the total volume of oxygen consumed by small organisms during respiration. The data obtained for volume would then be used to calculate the metabolic rate.
Procedure
Six tablets of potassium hydroxide (KOH) were placed at the bottom of a large test tube. A small separator that is permeable to carbon dioxide (CO2) was placed directly in front of the KOH pellets in order to protect the organism from the strong alkalinity of the solid KOH. Then, the mass of a small organism, either a cockroach or a cricket, was measured and then the organism was placed inside the test tube. A rubber stopper was fitted into the opening of the test tube and then a pipette was inserted into the opening in the rubber stopper. Parafilm was then wrapped around the top of the rubber stopper and around the bottom part of the pipette in order to create a water and airtight seal. This same setup was done for a control group with the exception of a living organism (plastic beads of around the same mass of the cricket were
used). In total, two experimental and two control respirometers were made.
After the setup for the respirometers was complete, the respirometers (both the controls and the experimentals) were placed in baths of water at two different temperatures. After letting the respirometers acclimate to the air pressure while in the bath, the respirometers were fully submerged and a timer was set after the water reached the first graduation. Every five minutes, data was recorded (see table 1).
Based on the gathered data, the rate of oxygen consumption was measured for each chamber. The rate was calculated by dividing the total volume of oxygen by time (see figure 1 and calculation section). This calculation was then converted from mL per minute to L per hour (see calculation section). The recorded mass of the organism was converted from grams to kilograms in order to determine the metabolic rate in L per hour per kilogram (see calculation section).
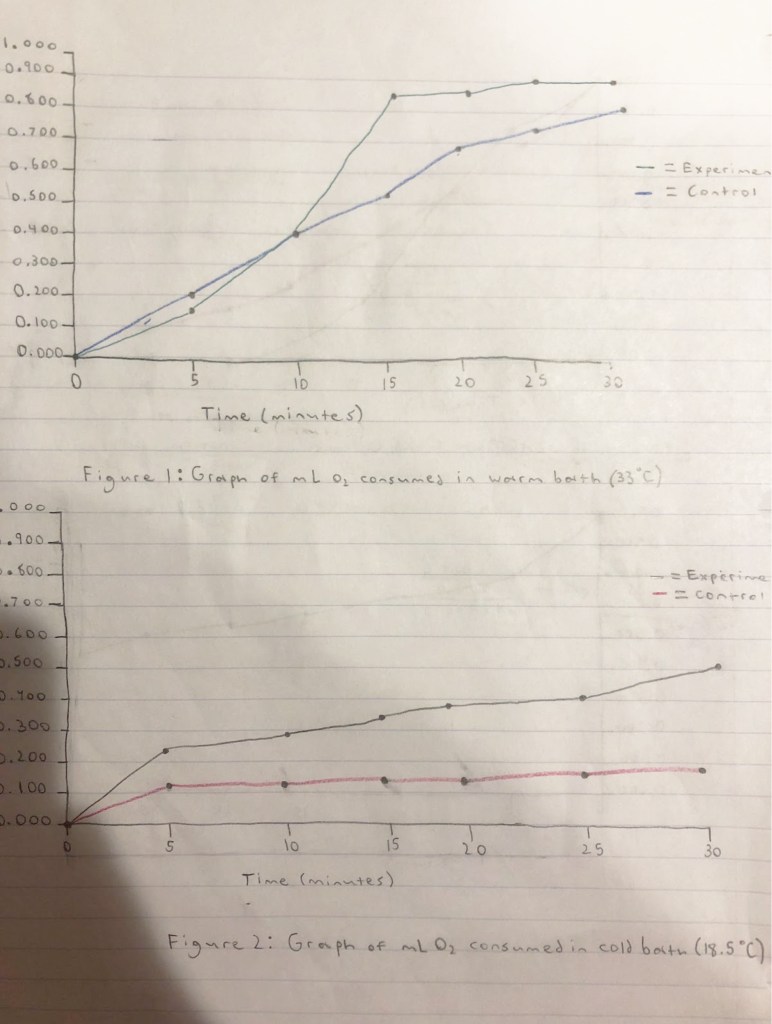
Data
Table 1: rate of oxygen consumption observed in warm water bath
| Experimental Chamber (Water Temperature: 33 C) (Organism Mass: 0.3 g) | Control Chamber (In same water bath as experimental) (Plastic Bead Mass: 0.3 g) |
| Time (min) | Reading at Time X (mL) | Initial Reading (mL) | Difference (mL) | Reading at time X (mL) | Initial Reading (mL) | Difference (mL) |
| 0 | 0.900 | ———— | 0.900 | ————— | ||
| 5 | 0.750 | 0.900 | 0.150 | 0.700 | 0.900 | 0.200 |
| 10 | 0.500 | 0.900 | 0.400 | 0.500 | 0.900 | 0.400 |
| 15 | 0.190 | 0.900 | 0.850 | 0.370 | 0.900 | 0.530 |
| 20 | 0.050 | 0.900 | 0.850 | 0.220 | 0.900 | 0.680 |
| 25 | 0.000 | 0.900 | 0.900 | 0.160 | 0.900 | 0.740 |
| 30 | 0.000 | 0.900 | 0.900 | 0.090 | 0.900 | 0.810 |
Table 2: Rate of Oxygen Consumption in Cold Water Bath
| Experimental Chamber (Water Temperature: 18.5 degrees C) (Organism Mass: 0.4 g) | Control Chamber (In same water bath as experimental) (Plastic Bead Mass: 0.4 g) |
| Time (min) | Reading at Time X (mL) | Initial Reading (mL) | Difference (mL) | Reading at time X (mL) | Initial Reading (mL) | Difference (mL) |
| 0 | 0.920 | ———— | 0.920 | ————— | ||
| 5 | 0.700 | 0.920 | 0.220 | 0.810 | 0.920 | 0.120 |
| 10 | 0.640 | 0.920 | 0.280 | 0.800 | 0.920 | 0.130 |
| 15 | 0.580 | 0.920 | 0.340 | 0.798 | 0.920 | 0.132 |
| 20 | 0.550 | 0.920 | 0.370 | 0.789 | 0.920 | 0.141 |
| 25 | 0.519 | 0.920 | 0.401 | 0.770 | 0.920 | 0.160 |
| 30 | 0.409 | 0.920 | 0.511 | 0.760 | 0.920 | 0.170 |
Calculations

Discussion
The data collected demonstrates that the warmer bath allowed for a faster reaction rate than the colder bath. This is consistent with the fundamentals of kinetics: temperature is proportional to the speed of reactants which leads to more reactant collisions. More reactant collisions means more reactions between particles in a given time frame. The effects of temperature on reactants are the same for proteins, as long as the temperature is within range of functionality, increased temperature leads to an increase in substrate collisions with enzyme, thus the rate of metabolism increases.
Including control chambers in the experiment are essential to make sure that the decrease in oxygen volume is due to the living organism and not to another outside factor. If there is a large decrease in oxygen in the control chamber, this indicates that another factor, such as failure in creating an air-tight seal, is creating a decrease in oxygen and not the organism.
The purpose of KOH is to decrease the volume of gas in the respirometer and allow for water to move down the pipette. This is allowed because KOH reacts with CO2 to form a solid precipitate, K2CO3. The formation of solid precipitate decreases the total volume of gas and allows for water to move down the indicator.
In this experiment, temperature was a factor that was considered when observing the rate of respiration in a small organism. Proton concentration is another factor that affects the rate of metabolism. Metabolic processes are made possible by enzymes. The environment around enzymes need to be kept within a certain pH range in order for the enzyme to maintain functionality.
Another factor is the relative nutrition/diet of the organism prior to experimentation. Many enzymes require cofactors and coenzymes such as ions and vitamins, respectively. If an organism is not getting proper concentrations of cofactors/coenzymes in their diet, enzyme functionality may be decreased and thus, metabolic rate.
The diet of the organism being tested also has to include proper numbers of glucose. In the experiment, the relative amount of glucose in each of the cricket’s diets. Glucose is a reactant in cellular respiration and greater reactant concentration, in general, leads to greater reaction rate.