Purpose
This was a two part study with a purpose to observe different aspects of osmosis. The purpose of part one was to determine the permeability of different substances to dialysis tubes. The purpose of part two was to determine what concentration of sucrose created an isotonic environment for a certain potato mass and determine its water potential.
Procedure
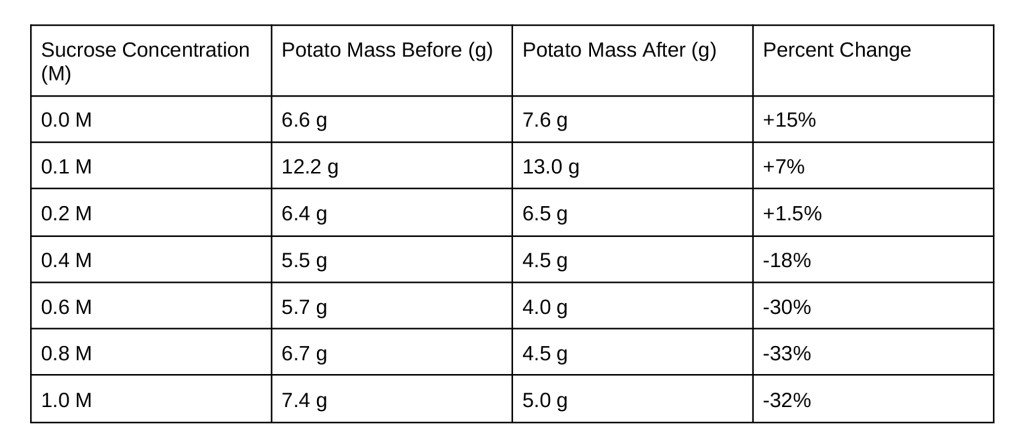
For part one, multiple student groups set up two open 500 mL beakers. One beaker was filled with tap-water and the other was filled with a solution of water and 1 M of either glucose, sucrose, or sodium chloride. Then two dialysis tubes were filled up, one was filled up with tap-water and the other was filled with a solution (referring to water and either sodium chloride, glucose, or sucrose).The dialysis tubes were weighed (in grams), closed with clamps and placed in beakers. The dialysis tube filled with water was put into the solution and the dialysis tube filled with water-substance solution was put into the beaker containing pure water. The beakers were then allowed to sit overnight and their results were evaluated the next day. The tubes were reweighed and their percent changes in weight were calculated (see table 1 in the data section).
For part two, multiple student groups set up one beaker containing a solution of water and different molar concentrations of sucrose (0.0 M, 0.1 M, 0.2 M, 0.4 M, 0.6 M, 0.8 M, and 1.0 M). Next, each group cut a piece of potato from a whole potato. The cut-out piece of potato was then weighed and placed in a certain solution. Each potato was left in the beaker overnight. The next day, each potato was weighed and their percent change was calculated (see table 2 in the data section). Using these measurements, the molar concentration of the isotonic environment was determined and then the water potential for the potato in that environment was then calculated using the equation for water potential: ψ = ψs + ψp , where ψs is the solute potential and ψp is the pressure potential. The equation for ψs =− 𝑖𝐶𝑅𝑇, where 𝑖 is the ionization constant, 𝐶 is the concentration (in M), 𝑅 is the universal gas constant, and 𝑇 is the temperature.
Data
Table 1: Weights and Percent Changes of dialysis tubes for Sodium Chloride (NaCl), Glucose, and Sucrose
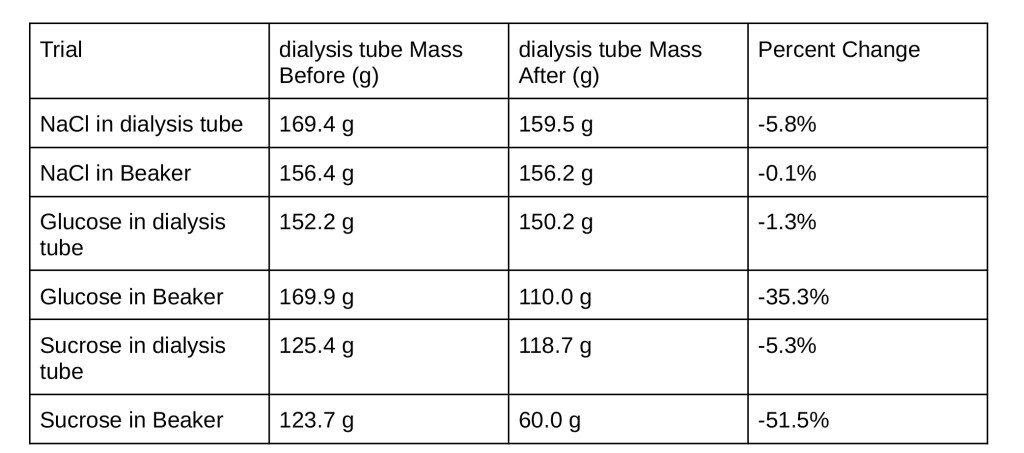
Table 2: Weights and Percent Changes of Potato Pieces for Different Molar Concentrations of Sucrose
Discussion
Part one: The expectation for the results was that the dialysis tube containing solution would increase in mass and the dialysis tube containing water would decrease in mass. This expectation is due to the concept of different tonicities. Water diffuses from lesser solute concentration to greater solute concentration. In a hypertonic environment, there is more solute outside of a cell (which is referring to anything with a semi-permeable membrane) so the cell loses mass due to the diffusion of water to the concentration of greater solute in the solution. In a hypotonic environment, there is greater solute concentration inside the cell than outside, so the cell will increase in mass. When the dialysis tube was filled with tap water inside of the beaker with solution that was a hypertonic environment. When the dialysis tube was filled with solution inside of a beaker filled with tap water, that was a hypotonic environment.
The solution in the beaker with water in the dialysis tube demonstrated expected results for a hypertonic environment: decrease in mass for the dialysis tube. However, when the solution was in the dialysis tube, the results showed a decrease in mass which is not consistent with what should happen to a cell in a hypotonic environment. All three solutions showed this result as a small percent decrease was calculated for all three.
Data shows that the dialysis tubing did serve as a selectively permeable membrane. Water was allowed to enter and exit the dialysis tubing, as the tubing was designed to do so. Water is polar and has a radius of about 0.27 nm. NaCl dissociates in water into a Na+ cation (0.19 nm radius) and a Cl- anion (0.175 nm radius). Both ions are smaller than water and polar, thus they can pass the membrane of the dialysis tubing. This is consistent with the data as the data shows a small percent change when solution was in the beaker (-0.1%) and in the dialysis tubing (-5.8%). Due to these small percent changes, it is concluded that a NaCl solution is isotonic.
The sucrose and glucose were not able to pass through the membrane, both are larger than water. This conclusion is consistent with the large percent decrease when a sucrose or glucose solution was in the beaker. When glucose was in the beaker there was a -35.5% change and when sucrose was in the beaker there was a -51.5% change. It was expected that when the sucrose or glucose solution was in the dialysis tube, the tube would gain mass. This was not the case however, the tube lost mass. This could be due to the pressure of the clamps on the tube, forcing water out. This same conclusion can be drawn for when NaCl solution was in the dialysis tube, even though NaCl was relatively isotonic, it also resulted in a loss of mass.
Part two: the apparent concentration of sucrose in a potato is 0.2 M. This is due to the fact that when a certain mass of potato is placed in a solution of 0.2 M sucrose, there is approximately a 1.5% increase in mass. This small change in mass is evidence that the potato was in a relatively isotonic environment in which the diffusion of water was in dynamic equilibrium. This is only possible if the concentrations of solute (in this case sucrose) are equal inside of the cell and outside.
As discussed, a relatively isotonic environment for a potato in a sucrose solution is 0.2 M and the water potential for a certain cell in an isotonic solution is approximately the same as the water potential for all other cells in that species. ψ = ψs + ψp . Since open beakers were used, ψp = 0, so ψ = ψs . Since sucrose was used and sucrose does not immediately dissociate like ionic compounds so 𝑖 = 1, 𝑐 = 0. 2 M, the universal gas constant for barr is 𝑅 = 0. 0831 𝐿𝑏𝑎𝑟𝑟/𝑚𝑜𝑙𝐾, and the temperature was 𝑇 = 293 𝐾. Plugging this in:
ψs = − (1)(0. 2𝑀)(0. 0831 𝐿𝑏𝑎𝑟𝑟/𝑚𝑜𝑙𝐾)(293𝑘) = − 4. 9 𝑏𝑎𝑟𝑟. -4.9 barr is the approximate water potential for a potato.
If the cylinders were allowed to dry out, meaning loss of water or dehydration, the water potential of the potato would change. Water potential is dependent on the concentration of the solution. If the cylinders are allowed to dry out this will change the concentration of both the water and the sucrose. The water concentration will decrease which will cause the sucrose concentration to increase. This is because sucrose is measured in moles per liter (mol/L) and thus the concentration of sucrose will increase because the denominator, liters, will decrease. This can also be represented as a decrease in dilution of water.
Water naturally diffuses from areas of lower solute concentration to higher solute concentration. In a hypotonic solution, in which the cell contains more solute than the surrounding cell, the water surrounding the cell will move into the cell because there is a higher concentration of solute inside. This results in an increase in cellular mass due to an increase in intracellular water. Too much gain in water will result in the lysing of an animal cell, but a hypotonic environment is ideal to maintain turgor pressure in plant cells.
When in a hypertonic solution, cells have lower solute concentration than the solution they are in, so water will leave the cell resulting in a net decrease in mass. This causes animal cells to crenelate and causes plant cells to undergo plasmolysis.
In an isotonic solution, the solute concentration is equal in both the cell and the surrounding solution. This results in dynamic equilibrium of the water diffusion. There will be nearly or no change in mass of the cell. Isotonic conditions are ideal for animal cells, but plant cells need turgor pressure because of their cell walls.
Humans cannot drink salt water for hydration because the concentration of salt in the water creates a hypertonic environment in cells. As discussed before, if too much salt water is taken in, cells will lose mass and crenelate which is not favorable for human cells to function properly.